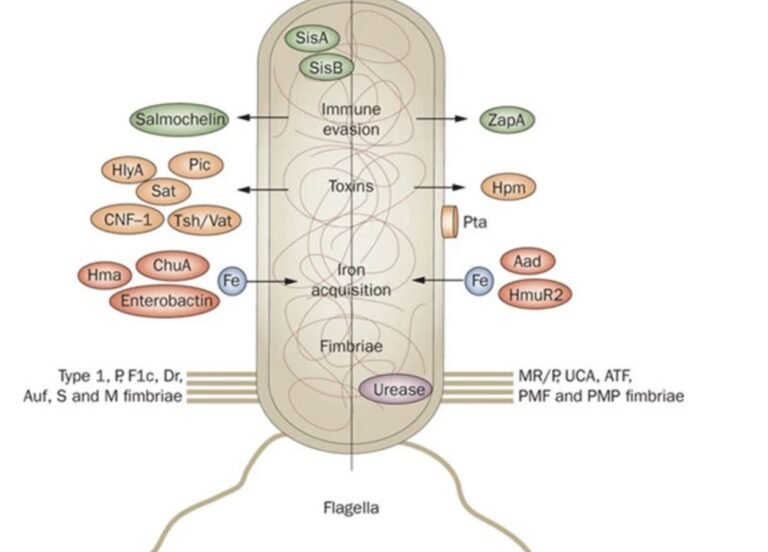
Escherichia coli (E. coli) O26, a pathogenic strain within the enterohemorrhagic E. coli (EHEC) group, has emerged as a significant cause of severe gastrointestinal diseases, including hemorrhagic colitis and hemolytic uremic syndrome (HUS). The severity of infections caused by E. coli O26 is influenced by the intricate interplay between host and pathogen. This article explores the host-pathogen interactions in E. coli O26 infections and their implications for disease severity, focusing on the roles of bacterial virulence factors, host immune responses, and genetic factors.
Bacterial Virulence Factors
E. coli O26 employs an arsenal of virulence factors to establish infection, evade host defenses, and cause disease. Some of the key virulence factors include:
- Shiga Toxins (Stx): Shiga toxins, particularly Stx1 and Stx2, are potent cytotoxins produced by E. coli O26. These toxins inhibit protein synthesis in host cells by cleaving a specific adenine residue in the 28S rRNA of the ribosome. The resultant cellular damage leads to apoptosis and necrosis, contributing to the characteristic bloody diarrhea and potential systemic complications such as HUS. The type and amount of Shiga toxins produced can significantly influence disease severity.
- Type III Secretion System (T3SS): The T3SS is a needle-like apparatus that injects bacterial effector proteins directly into host cells. These effectors modulate various host cell functions, including cytoskeletal rearrangements, apoptosis, and immune responses. In E. coli O26, the T3SS facilitates intimate attachment to the intestinal epithelium, forming attaching and effacing (A/E) lesions. These lesions disrupt the microvilli, leading to diarrhea and facilitating bacterial colonization.
- Adhesins: Adhesins are surface proteins that mediate bacterial adherence to host tissues. E. coli O26 utilizes various adhesins, such as intimin, to bind to host cell receptors. Intimin, encoded by the eae gene, interacts with the translocated intimin receptor (Tir), an effector protein injected into the host cell via T3SS. This interaction strengthens the bacteria-host cell attachment and contributes to A/E lesion formation.
Host Immune Responses
The host immune response plays a crucial role in determining the outcome of E. coli O26 infections. The interaction between bacterial virulence factors and host immune defenses can influence disease severity.
- Innate Immune Response: Upon infection, the host’s innate immune system is activated to recognize and eliminate the invading pathogen. Pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs), detect pathogen-associated molecular patterns (PAMPs) and trigger an inflammatory response. This response involves the recruitment of immune cells, production of cytokines, and activation of antimicrobial mechanisms. However, excessive inflammation can cause tissue damage and exacerbate disease severity.
- Adaptive Immune Response: The adaptive immune system generates a specific response to E. coli O26 infection, involving the activation of B and T lymphocytes. B cells produce antibodies that neutralize bacterial toxins and facilitate pathogen clearance, while T cells help orchestrate the immune response. The effectiveness of the adaptive immune response can influence the resolution of infection and the prevention of complications like HUS.
- Immune Evasion: E. coli O26 employs various strategies to evade the host immune response. Shiga toxins suppress the host immune system by inducing apoptosis in macrophages and dendritic cells. The T3SS effectors also modulate host signaling pathways to dampen inflammation and promote bacterial survival. These evasion mechanisms can prolong infection and increase disease severity.
Genetic Factors
Host genetic factors can significantly influence susceptibility to E. coli O26 infections and disease outcomes.
- Genetic Predisposition: Certain genetic polymorphisms in host immune genes can affect the susceptibility to E. coli O26 infections and the severity of disease. For example, variations in the genes encoding TLRs and cytokines can influence the strength and effectiveness of the immune response. Individuals with specific genetic profiles may be more prone to severe disease manifestations.
- HLA Typing: The human leukocyte antigen (HLA) system plays a critical role in the adaptive immune response by presenting antigens to T cells. HLA typing can influence the ability of the immune system to recognize and respond to E. coli O26 antigens. Certain HLA types may be associated with increased susceptibility to severe disease.
Implications for Disease Severity
The complex interplay between E. coli O26 virulence factors, host immune responses, and genetic factors determines the severity of infection. Understanding these interactions is essential for developing effective strategies to prevent and treat E. coli O26 infections.
- Early Diagnosis and Intervention: Timely diagnosis and intervention are crucial for managing E. coli O26 infections and preventing complications. Identifying individuals at higher risk of severe disease based on genetic and immunological factors can guide targeted interventions and improve outcomes.
- Vaccine Development: Developing vaccines that target key virulence factors of E. coli O26, such as Shiga toxins and T3SS components, holds promise for preventing infections and reducing disease severity. Vaccination strategies should consider host genetic variability to enhance effectiveness.
- Therapeutic Approaches: Novel therapeutic approaches that modulate the host immune response or neutralize bacterial virulence factors are needed to combat E. coli O26 infections. Immunotherapy, monoclonal antibodies, and small-molecule inhibitors targeting specific host-pathogen interactions are potential avenues for treatment.
Conclusion
The severity of E. coli O26 infections is influenced by the intricate interplay between bacterial virulence factors, host immune responses, and genetic factors. Understanding these host-pathogen interactions is essential for developing effective strategies to prevent and treat infections, ultimately reducing the impact of this pathogen on public health. Continued research in this field will provide valuable insights into the mechanisms underlying disease severity and inform the development of targeted interventions.